COVID19: N720D AN ACE2 VARIANT WITH HIGHER AFFINITY TO TMPRSS2
Published on 09/10/2020
The rate of infection and mortality of SARS-CoV-2 varies based on the geographical spread of the virus as a consequence of several factors such as isolation, quarantine, differences in the genetic makeup of various populations, and mutations in the SARS-CoV-2 genome. A recent publication by a team at DDI led by Prof. Fahd Al-Mulla titled, “A comprehensive germline variant and expression analyses of ACE2, TMPRSS2 and SARS-CoV-2 activator FURIN genes from the Middle East: Combating SARS-CoV-2 with precision medicine” depicted two variations in the angiotensin-converting enzyme 2 (ACE2) protein, which is vital for SARS-CoV-2 entry into human cells via its spike protein. These variations were more prevalent in Europeans than in Middle Eastern populations. In addition, it is thought that the variants can be responsible for an increase in viral infectivity and viral load.
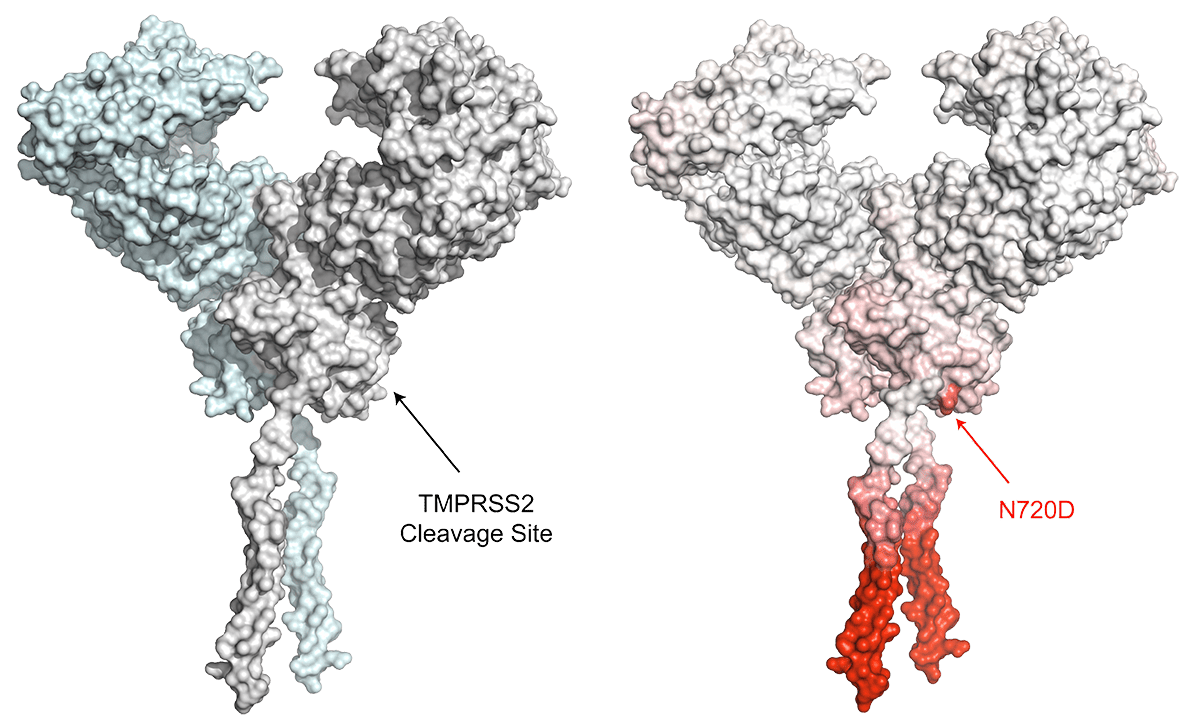
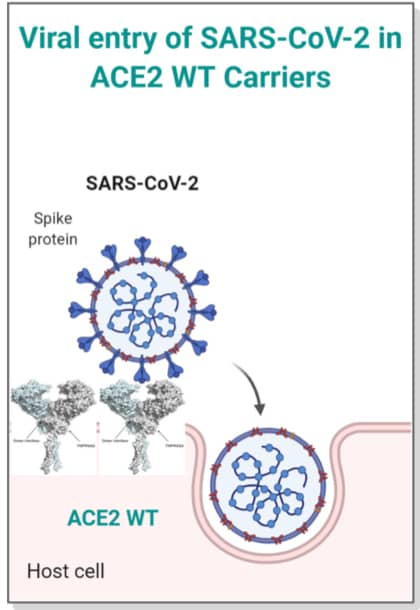
To be functional, ACE2 needs to be cleaved by an enzyme called type II transmembrane serine protease (TMPRSS2). Since one of the ACE2 genetic variants (N720D) is close to TMPRSS2 cleavage site, any structural changes caused by N720D might affect this process. We used computational structural biology tools to predict the effect of the N720D variant on the stability and flexibility of ACE2 structure. Furthermore, we modelled the binding of TMPRSS2 to wild type ACE2 and the N720D variant. Based on our modelling analysis, we postulate that this variant impacts the stability and flexibility of ACE2, resulting in a more favorable site for TMPRSS2 binding and cleavage.
As a result, carriers of this variant possibly have an increased S-protein binding and can potentially have increased viral entry caused by enhanced interaction between ACE2 and TMPRSS2. This research helps us understand the differences in the severity of COVID-19 among different patients and confirms the role of genetics in such variations. However, more functional in-vitro studies are required to corroborate our findings.
Anwar Mohammad, Sulaiman K. Marafie, Eman Alshawaf, Mohamed Abu-Farha, Jehad Abubaker, Fahd Al-Mulla. Structural analysis of ACE2 variant
N720D demonstrates a higher binding affinity to TMPRSS2. Life sciences. Volume 295, 15 October 2020
https://doi.org/10.1016/j.lfs.2020.118219


